Cell and Gene Therapies: Identifying and Tracking Total Costs of Treatment
Single-administration cell and gene therapies cost hundreds of thousands to $4.25 million, but that isn't the total treatment cost. How can the total cost be identified and monitored in claims data?
There are currently 30+ FDA approved cell and gene therapies (CGTs), ranging from a list price of a few hundred thousand to $4.25 million. However, that's just the cost of the drug. Many of these drugs require other extensive procedures associated with drug administration and ongoing patient monitoring.
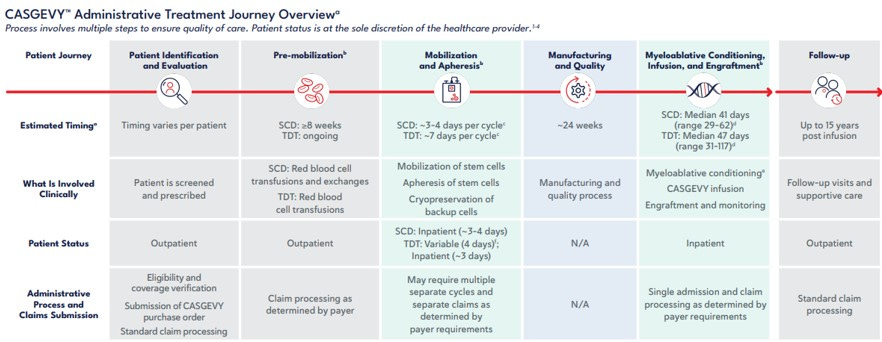
For example, the sickle cell disease CGTs, Casgevy and Lyfgenia, require patients to go through multiple steps of varying intensity and length over several months before returning to the hospital to finally be administered the drug inpatient and then monitored for a few more months. None of the costs of preparing and administering the drug or monitoring the patient are included in the $2.2 million or $3.1 million cost of the drug. See the image below for the full process.
Source: https://www.casgevyhcp.com/sites/default/files/coding-and-billing-guide.pdf
The amount of associated costs will vary depending on the CGT. For CGTs with outpatient administration, it could be a few hundred dollars. For those that require extensive inpatient stays at a hospital, the associated costs could be hundreds of thousands of dollars added to the cost of the drug.
This variability can make it very difficult for payers to estimate their cost exposure for covering CGTs. For in-market therapies, it may be possible to identify these costs in insurance claims. However, identifying the full scope of the episode can still be difficult, given the various procedures, monitoring, and potential complications associated with treatment. These issues are compounded for new CGTs or those for ultra rare diseases. It's important to have someone that understands the full process of receiving CGT treatment to estimate the additional associated costs.
Identify CGTs in claims
Being able to identify CGTs in claims data will be vital for evaluating and estimating the impact on a payer. It may be helpful to create a process and CGT reference now while the number of CGTs is still manageable. For the newer CGTs, here’s a good general process:
Start with the billing guides from the manufacturers—these are publicly available.
Identify the CGT with ICD-10 procedure codes or HCPCS codes.
If the ICD-10 doesn’t have the NDC included, you’ll need to apply a little more scrutiny to make sure it’s the right claim to include in your search.
If the drug has multiple indications, include the desired indication via ICD-10 diagnosis code(s).
Choose the episode window to look back and forward in the claims from the administration date.
This depends on the specific CGT, but most are 30-90 days. The sickle cell disease treatments can be as long as 12 months.
For unclassified HCPCS codes, check NDCs.
If NDC isn’t available, you may consider looking for other signals that a CAR-T therapy was started, such as apheresis.
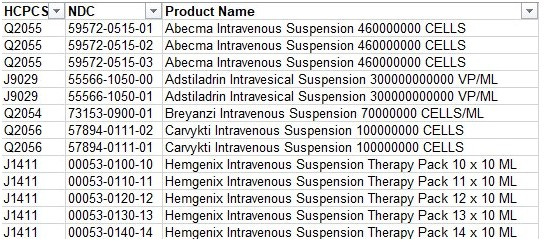
If you're wanting to find CGTs in your claims, you should compile a list of the identifiers like those below. The exact data you need depends on the place of service that the patients receive the CGT, so the identifiers by place of service below may be useful.
Inpatient: ICD-10 procedure codes
Outpatient: J or Q codes
Pharmacy: NDC
Here’s a small sample of CGT identifiers as an example:
Some of this information is publicly available, but not sourced in one place for easy reference on CGTs. A few nuances to mention:
J/Q codes may not be specific to the CGT, particularly if it is a very new CGT (e.g., some are J3590 = unclassified biologic)
It is good to confirm the drug with NDC, if available
There are often multiple NDCs for each CGT
Sometimes part of the treatment will be inpatient and the rest will be outpatient/home health (see Casgevy example above)
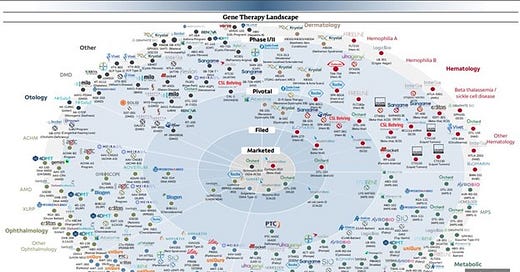
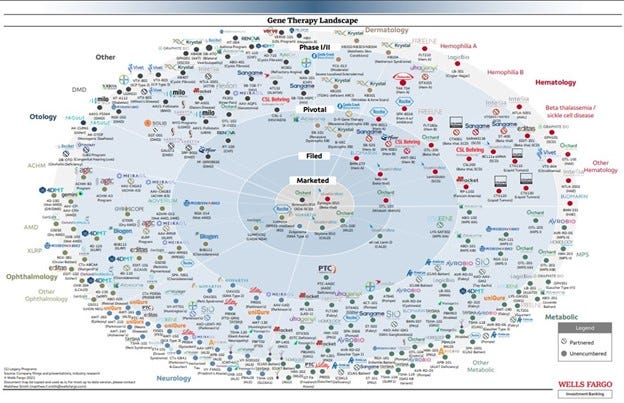
As CGT utilization increases from more CGT FDA approvals (see below for a CGT pipeline visual made by Wells Fargo in 2022 to get an idea of the landscape) as well as improved access to administration sites (here are the current sites for Casgevy) and familiarity with the therapies, the information above should become even more valuable for identifying CGTs in claims and estimating total cost exposure.
Click here for a larger view of the visual



Great article!